Ep Reference Standard Catalogue. Get more information about IP Botanical Reference Standards by. The following list of RSs have been assigned a Valid Use Date of October 31 2020. She will take over the function from the current Director Susanne Keitel in October 2021. The European Directorate for the Quality of Medicines HealthCare EDQM announces the availability of 2 new European Pharmacopoeia Ph.
 Usp Ep Ip Bp Reference Standard Usp Reference Standards Wholesale Trader From Ankleshwar
Usp Ep Ip Bp Reference Standard Usp Reference Standards Wholesale Trader From Ankleshwar From indiamart.com
Usp Ep Ip Bp Reference Standard Usp Reference Standards Wholesale Trader From Ankleshwar
Usp Ep Ip Bp Reference Standard Usp Reference Standards Wholesale Trader From Ankleshwar From indiamart.com
More related: Ford Trucks For Sale Massachusetts - General Motors Lake Orion Assembly - Census 2020 Interactive Map - Great Wall Restaurant Denver -
The Council of Europe has appointed Petra Dörr PhD as future Director of the European Directorate for the Quality of Medicines HealthCare EDQM. 2- Name lists the name of each item as designated in the European Pharmacopoeia English version andor on. Get more information about EP Reference Standards by downloading the latest brochure. 46 of C 22 H 24 ClN 2 O 8. Desired quantity for item in this row. Families of exclusive compounds.
Desired quantity for item in this row.
The European Directorate for the Quality of Medicines HealthCare EDQM announces the availability of 2 new European Pharmacopoeia Ph. International chemical reference standards - ICRS. Once a current lot is depleted it becomes the previous lot At this time a valid use date is assigned which is typically 3-12 months from the. European Pharmacopeia Reference Standards EP The European Directorate for the Quality of Medicines and HealthCare EDQM is a leading organisation that protects public health by developing and monitoring the application of quality standards for safe medicines and their safe use. The Shipping group column shows you the shipping temperature for. Reference Standard Collection Catalogue News 06 September 2021 Strasbourg France.
 New Versatile Reference Standard For Equipment Qualification Edqm European Directorate For The Quality Of Medicines
Source: edqm.eu
New Versatile Reference Standard For Equipment Qualification Edqm European Directorate For The Quality Of Medicines
Source: edqm.eu
580 which will become official November 1 2020 users will no longer be able to assume a value of 1000 for these RS lots in quantitative USP compendial.
 Fusidic Acid European Pharmacopoeia Ep Reference Standard 6990 06 3
Source: sigmaaldrich.com
Fusidic Acid European Pharmacopoeia Ep Reference Standard 6990 06 3
Source: sigmaaldrich.com
The Shipping group column shows you the shipping temperature for.
2
Source:
The Shipping group column shows you the shipping temperature for.
 5o8pvxeyxycy5m
Source:
5o8pvxeyxycy5m
Source:
International standards for antibiotics ISA.
Usp Reference Standard Endotoxin E700 Rse Lonza
Source: bioscience.lonza.com
Desired quantity for item in this row.
 European Pharmacopoeia
Source: yumpu.com
European Pharmacopoeia
Source: yumpu.com
580 which will become official November 1 2020 users will no longer be able to assume a value of 1000 for these RS lots in quantitative USP compendial.
 Reference Standards Of European Pharmacopoeia Edqm
Source: edqm.eu
Reference Standards Of European Pharmacopoeia Edqm
Source: edqm.eu
Reference Standard Collection Catalogue News 06 September 2021 Strasbourg France.

1- Catalogue Code designates the catalogue code that has been assigned to each Reference Standard.
 Indian Pharmacopeia Reference Standards Ip Are The Official Standards Issued By The Indian Pharmacopoeia Commission Ipc
Source: chromachemie.co.in
Indian Pharmacopeia Reference Standards Ip Are The Official Standards Issued By The Indian Pharmacopoeia Commission Ipc
Source: chromachemie.co.in
Part of ISO 17025 Accreditation.
 Reference Materials Epichem
Source: epichem.com.au
Reference Materials Epichem
Source: epichem.com.au
Once you have sent your order or your quotation request you will receive an official order confirmationquotation within 48 hours.
 Usp Ep Ip Bp Reference Standard Usp Reference Standards Wholesale Trader From Ankleshwar
Source: indiamart.com
Usp Ep Ip Bp Reference Standard Usp Reference Standards Wholesale Trader From Ankleshwar
Source: indiamart.com
The Shipping group column shows you the shipping temperature for.
 Chromachemie Is An Authorised Distributor For European Pharmacoepia Reference Standards In India
Source: chromachemie.co.in
Chromachemie Is An Authorised Distributor For European Pharmacoepia Reference Standards In India
Source: chromachemie.co.in
The reference will however remain available for sale for a further 6 months subject to stock availability ie.
Reference Standards Of European Pharmacopoeia Edqm
Source: edqm.eu
As of July 2021 EDQM reference spectra will be sent exclusively.
2
Source:
46 of C 22 H 24 ClN 2 O 8.
 Other Pharmacopeial Standards
Source: chromachemie.co.in
Other Pharmacopeial Standards
Source: chromachemie.co.in
The Council of Europe has appointed Petra Dörr PhD as future Director of the European Directorate for the Quality of Medicines HealthCare EDQM.
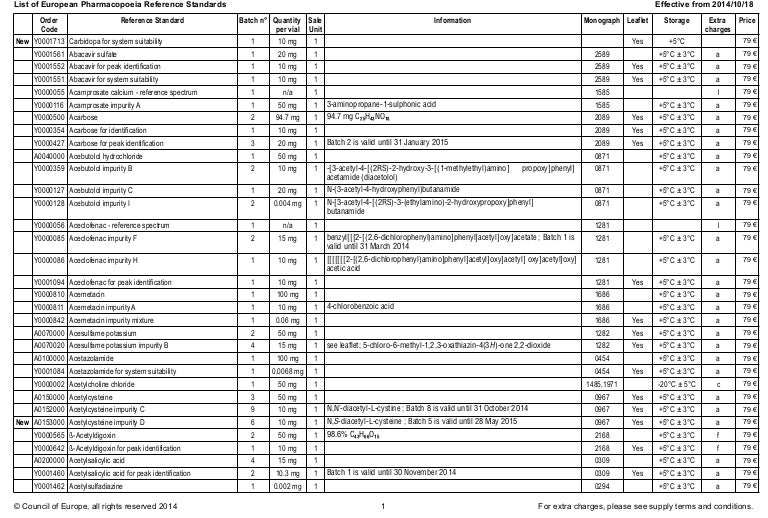 European Pharmacopoeia Rs Catalog Crs
Source: slideshare.net
European Pharmacopoeia Rs Catalog Crs
Source: slideshare.net
The reference will remain visible in the catalogue until 01012022.
 Usp Ep Ip Bp Reference Standard Usp Reference Standards Wholesale Trader From Ankleshwar
Source: indiamart.com
Usp Ep Ip Bp Reference Standard Usp Reference Standards Wholesale Trader From Ankleshwar
Source: indiamart.com
Desired quantity for item in this row.
 European Pharmacopoeia Reference Standards Released In February 2018 Edqm European Directorate For The Quality Of Medicines
Source: edqm.eu
European Pharmacopoeia Reference Standards Released In February 2018 Edqm European Directorate For The Quality Of Medicines
Source: edqm.eu
Ep 2014 reference standards catalogue.
