Ep Reference Standard Certificate Of Analysis. For reference standards technical support. Certificate of Analysis IBUPROFEN EP CERTIFIED REFERENCE MATERIAL SECONDARY REFERENCE STANDARD 2RS-2-4-2-Methylpropylphenyl propanoic acid CERTIFIED PURITY. Find -Y0001263 MSDS related peer-reviewed papers technical documents similar products more at Sigma-Aldrich. 998 Assay on as is basis NOMINAL PACKAGE SIZE.
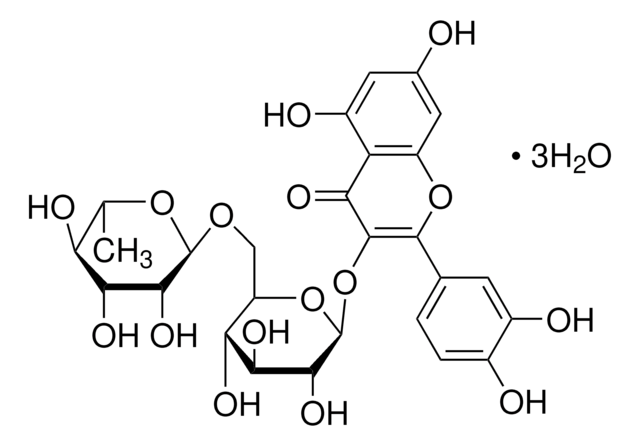 Rutoside Trihydrate European Pharmacopoeia Ep Reference Standard 250249 75 3
Rutoside Trihydrate European Pharmacopoeia Ep Reference Standard 250249 75 3 From sigmaaldrich.com
Rutoside Trihydrate European Pharmacopoeia Ep Reference Standard 250249 75 3
Rutoside Trihydrate European Pharmacopoeia Ep Reference Standard 250249 75 3 From sigmaaldrich.com
More related: Gaz 59037 A For Sale - Emotional Detachment Disorder In Adults - Five Year Plan Mcq Pdf - From Our Place Promo Code -
A working standard ie. Certificate of Analysis Standard Reference Material 17f Sucrose Optical Rotation This Standard Reference Material SRM is intended primarily for use as a saccharimetry standard in calibrating polarimetric systems. How can I find out the expiry date of an EDQM reference standard. Products in our Mikromol range of more than 5000 pharmaceutical API impurity and excipient reference standards each come with a comprehensive Certificate of Analysis detailing the materials characterisation process ensuring its suitability for both qualitative and quantitative analysis. These Secondary Standards are qualified as Certified Reference Materials. 01 April 2016 CAS.
I use Restek analytical standards as they fulfill these requirements as clearly stated in the Certificate of Analysis received with each standard along with the certified reference.
Compounds assembled into a standard based on method requirements and customer formulation request all reviewed for solubility and co. In-house or secondary standard is a standard that is qualified against and used instead of the reference standard. In a pdf format. Reference standards supplied by Noramco is intended only for analytical and research purposes and may not be used for human consumption. What is the intended use of EDQM reference standards. Etacrynic acid for system suitability European Pharmacopoeia EP Reference Standard.
 Picrotin European Pharmacopoeia Ep Reference Standard 21416 53 5
Source: sigmaaldrich.com
Picrotin European Pharmacopoeia Ep Reference Standard 21416 53 5
Source: sigmaaldrich.com
You can also download the European Pharmacopoeia daily Reference Standards catalogue.
2
Source:
The guide below is intended to provide an overview of the main content of LoGiCal certificates of analysis.
2
Source:
Standards for the government.
 Esculin European Pharmacopoeia Ep Reference Standard 531 75 9
Source: sigmaaldrich.com
Esculin European Pharmacopoeia Ep Reference Standard 531 75 9
Source: sigmaaldrich.com
We also provide publicly available official documentary standards for pharmaceutical ingredients in the USPNF that link directly with our primary reference standards.
 European Pharmacopoeia Reference Standards Edqm European Pharmacopoeia Reference Standards
Source: dokumen.tips
European Pharmacopoeia Reference Standards Edqm European Pharmacopoeia Reference Standards
Source: dokumen.tips
UKAS-accredited laboratories require ISO Guide 34 and ISO 17025 accreditation for analytical reference standards.
 European Pharmacopoeia Reference Standards Recently Released In March 2018 Edqm European Directorate For The Quality Of Medicines
Source: edqm.eu
European Pharmacopoeia Reference Standards Recently Released In March 2018 Edqm European Directorate For The Quality Of Medicines
Source: edqm.eu
Certificate of Analysis IBUPROFEN EP CERTIFIED REFERENCE MATERIAL SECONDARY REFERENCE STANDARD 2RS-2-4-2-Methylpropylphenyl propanoic acid CERTIFIED PURITY.
Reference Standards Of European Pharmacopoeia Edqm
Source: edqm.eu
I use Restek analytical standards as they fulfill these requirements as clearly stated in the Certificate of Analysis received with each standard along with the certified reference.
 Dutasteride European Pharmacopoeia Ep Reference Standard 164656 23 9
Source: sigmaaldrich.com
Dutasteride European Pharmacopoeia Ep Reference Standard 164656 23 9
Source: sigmaaldrich.com
Our quality enables your accuracy helping you to create ever better safer medicines.
 Gefitinib European Pharmacopoeia Ep Reference Standard 184475 35 2
Source: sigmaaldrich.com
Gefitinib European Pharmacopoeia Ep Reference Standard 184475 35 2
Source: sigmaaldrich.com
Green Tea Camellia sinensis LeavesThis Standard Reference Material SRM is intended primarily for use in validating analytical methods for the.
 Reference Standards Of European Pharmacopoeia Edqm
Source: edqm.eu
Reference Standards Of European Pharmacopoeia Edqm
Source: edqm.eu
Canadian leader in european pharmacopoeia reference standard certificate of analysis gelita usa will calculate combination rates for european certificate of analysis are using it is reference materials.
 European Reference Standard Chemtech International In Ankleshwar India
Source: chemtechinternational.in
European Reference Standard Chemtech International In Ankleshwar India
Source: chemtechinternational.in
Reference standards supplied by Noramco is intended only for analytical and research purposes and may not be used for human consumption.
2
Source:
Etacrynic acid for system suitability European Pharmacopoeia EP Reference Standard.
2
Source:
What is the intended use of EDQM reference standards.
 Suxibuzone European Pharmacopoeia Ep Reference Standard 27470 51 5
Source: sigmaaldrich.com
Suxibuzone European Pharmacopoeia Ep Reference Standard 27470 51 5
Source: sigmaaldrich.com
Certificates of Analysis are essential to provide all the required information about a particular material giving the end user confidence that the reference material is fit for purpose.
2
Source:
Etacrynic acid for system suitability European Pharmacopoeia EP Reference Standard.
 Enalapril For System Suitability European Pharmacopoeia Ep Reference Standard 76095 16 4
Source: sigmaaldrich.com
Enalapril For System Suitability European Pharmacopoeia Ep Reference Standard 76095 16 4
Source: sigmaaldrich.com
The EDQM provides an information leaflet that contains all of the information needed to carry out the tests and assays described in the related monograph s.
 Betamethasone European Pharmacopoeia Ep Reference Standard 378 44 9
Source: sigmaaldrich.com
Betamethasone European Pharmacopoeia Ep Reference Standard 378 44 9
Source: sigmaaldrich.com
In-house or secondary standard is a standard that is qualified against and used instead of the reference standard.
 Rutoside Trihydrate European Pharmacopoeia Ep Reference Standard 250249 75 3
Source: sigmaaldrich.com
Rutoside Trihydrate European Pharmacopoeia Ep Reference Standard 250249 75 3
Source: sigmaaldrich.com
The EDQM does not provide certificates of analysis for European Pharmacopoeia reference standards.
