Ep Reference Standard List. If you wish to modify or cancel your request please send an email to ordersedqmeu. USP regularly tests the standards you use to ensure that they remain up-to-date and suitable for your compendial needs. Receive notifications based on your needs and usage by downloading the USP Reference Standards App today. If there are questions on whether a source of a standard would be considered by FDA to be an official source applicants should contact the appropriate chemistry review staff.
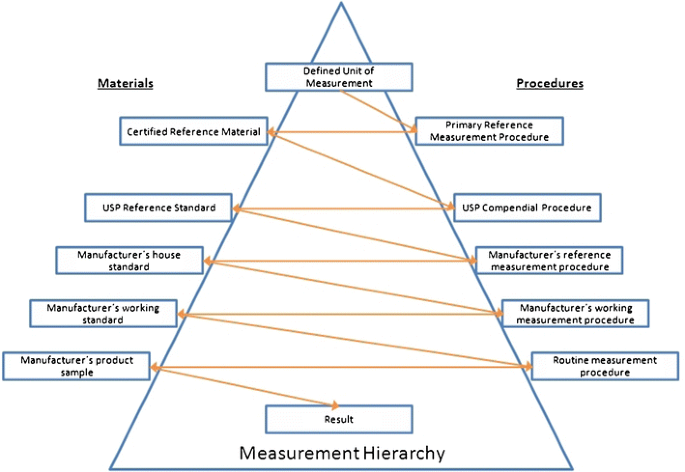 Primary And Secondary Reference Materials For Procedures To Test The Quality Of Medicines And Foods Springerlink
Primary And Secondary Reference Materials For Procedures To Test The Quality Of Medicines And Foods Springerlink From link.springer.com
Primary And Secondary Reference Materials For Procedures To Test The Quality Of Medicines And Foods Springerlink
Primary And Secondary Reference Materials For Procedures To Test The Quality Of Medicines And Foods Springerlink From link.springer.com
More related: Emotional Quotes In Hindi On Life - From Russia With Love Psp Cheats - Dr Friend Conway Ar - Cv Format Template Bd -
Once you have sent your order or your quotation request you will receive an official order confirmationquotation within 48 hours. Orders or quotation requests can be submitted to the EDQM. Indian Pharmacopeia Reference Standards IP IP Reference Substances abbreviated to IPRS and referred to as RS in the individual monographs are the official standards issued by the Indian Pharmacopoeia Commission IPC. Reference for EP European Pharmacopoeia Liquid Color Standards. Please submit each order or request only once either via the WebStore or by e-mail. Please enter a search term and select a search method using the drop menus below.
Reference Standard Collection Catalogue News 06 September 2021 Strasbourg France.
Once you have sent your order or your quotation request you will receive an official order confirmationquotation within 48 hours. By e-mail to ordersedqmeu. Reference for EP European Pharmacopoeia Liquid Color Standards. Eur reference standards and 15 replacement batches for Ph. All Reference Standards New Reference Standards Upcoming Small Molecules chevron_right Biologics chevron_right Dietary Supplements and Herbal Medicines chevron_right Excipients chevron_right Foods chevron_right General Chapters chevron_right Impurities Related Compounds chevron_right Dissolution US DEA Regulated Items. Welcome to the EDQM reference standards ordering website.
 Catalogues Publications Lgc Standards
Source: lgcstandards.com
Catalogues Publications Lgc Standards
Source: lgcstandards.com
Desired quantity for item in this row.
 Indian Pharmacopeia Reference Standards Ip Are The Official Standards Issued By The Indian Pharmacopoeia Commission Ipc
Source: chromachemie.co.in
Indian Pharmacopeia Reference Standards Ip Are The Official Standards Issued By The Indian Pharmacopoeia Commission Ipc
Source: chromachemie.co.in
862 of C 7 H 10 NNaO 7 P 2.
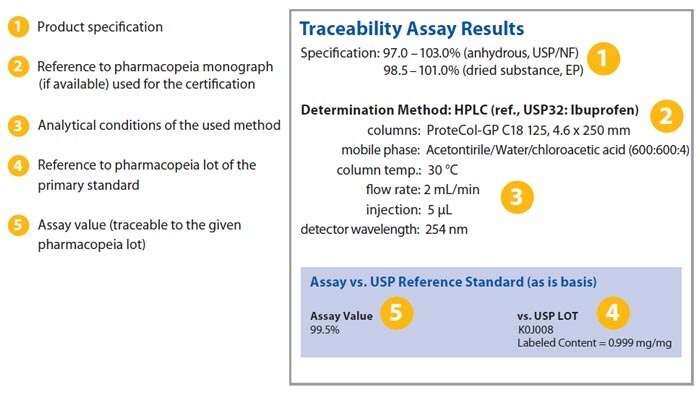 Pharmaceutical Secondary Standards
Source: sigmaaldrich.com
Pharmaceutical Secondary Standards
Source: sigmaaldrich.com
The European Directorate for the Quality of Medicines HealthCare EDQM announces the availability of 2 new European Pharmacopoeia Ph.
 European Pharmacopoeia Ph Eur 10th Edition Edqm European Directorate For The Quality Of Medicines
Source: edqm.eu
European Pharmacopoeia Ph Eur 10th Edition Edqm European Directorate For The Quality Of Medicines
Source: edqm.eu
COA of IPRS IPRS and Impurity Standards Roadmap for 2016-17 825KB.
2
Source:
USP regularly tests the standards you use to ensure that they remain up-to-date and suitable for your compendial needs.
 Primary And Secondary Reference Materials For Procedures To Test The Quality Of Medicines And Foods Springerlink
Source: link.springer.com
Primary And Secondary Reference Materials For Procedures To Test The Quality Of Medicines And Foods Springerlink
Source: link.springer.com
Eur reference standards and 15 replacement batches for Ph.
 Reference Standards Of European Pharmacopoeia Edqm
Source: edqm.eu
Reference Standards Of European Pharmacopoeia Edqm
Source: edqm.eu
How to place an order.
 5o8pvxeyxycy5m
Source:
5o8pvxeyxycy5m
Source:
It may be possible to use a USP RS outside of its associated USP compendial applications.
 Reference Standards Of European Pharmacopoeia Edqm
Source: edqm.eu
Reference Standards Of European Pharmacopoeia Edqm
Source: edqm.eu
Indian Pharmacopeia Reference Standards IP IP Reference Substances abbreviated to IPRS and referred to as RS in the individual monographs are the official standards issued by the Indian Pharmacopoeia Commission IPC.
 European Pharmacopoeia Wikipedia
Source: en.wikipedia.org
European Pharmacopoeia Wikipedia
Source: en.wikipedia.org
Eur reference standards and 15 replacement batches for Ph.
Reference Standards Of European Pharmacopoeia Edqm
Source: edqm.eu
USP Reference Standards are specified for use in conducting official USPNF tests and assays.
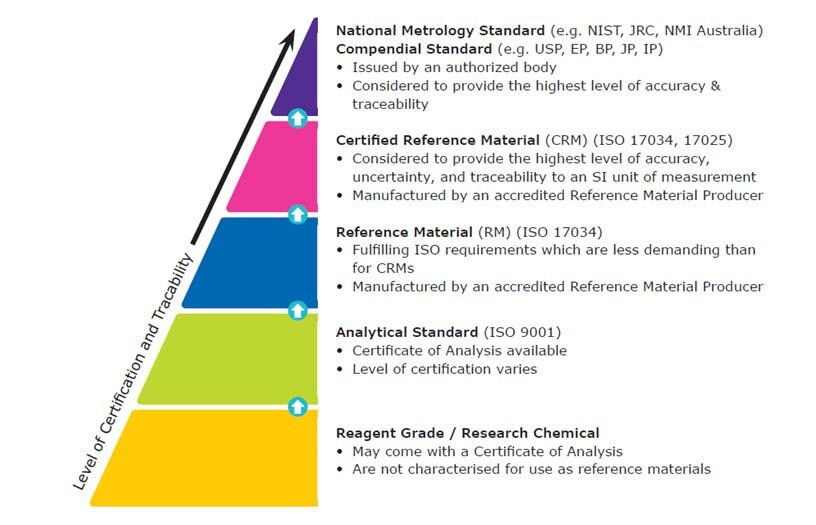 Calibration Qualification Validation
Source: sigmaaldrich.com
Calibration Qualification Validation
Source: sigmaaldrich.com
European Pharmacopoeia Method 222 Degree of Coloration of Liquids European Pharmacopoeia Strasbourg France 1997.
 Chemical Reference Standards Released In Support Of European Pharmacopoeia Eu Science Hub
Source: ec.europa.eu
Chemical Reference Standards Released In Support Of European Pharmacopoeia Eu Science Hub
Source: ec.europa.eu
Chromachemie is a supplier of impurity standards in india.
 How To Choose The Correct Reference Material Quality Grade
Source: sigmaaldrich.com
How To Choose The Correct Reference Material Quality Grade
Source: sigmaaldrich.com
Reference Standard Collection Catalogue News 06 September 2021 Strasbourg France.
 Solubility Determination Of Active Pharmaceutical Ingredients Which Have Been Recently Added To The List Of Essential Medicines In The Context Of The Biopharmaceutics Classification System Biowaiver Journal Of Pharmaceutical Sciences
Source: jpharmsci.org
Solubility Determination Of Active Pharmaceutical Ingredients Which Have Been Recently Added To The List Of Essential Medicines In The Context Of The Biopharmaceutics Classification System Biowaiver Journal Of Pharmaceutical Sciences
Source: jpharmsci.org
The European Directorate for the Quality of Medicines HealthCare EDQM announces the availability of 2 new European Pharmacopoeia Ph.
 European Pharmacopoeia 8 0 Pdf Download Higgs Tours Ocho Rios Jamaica
Source: higgs-tours.ning.com
European Pharmacopoeia 8 0 Pdf Download Higgs Tours Ocho Rios Jamaica
Source: higgs-tours.ning.com
USP Reference Standards are specified for use in conducting official USPNF tests and assays.
 Imipramine 99 Tlc 113 52 0 Sigma Aldrich
Source: sigmaaldrich.com
Imipramine 99 Tlc 113 52 0 Sigma Aldrich
Source: sigmaaldrich.com
USP Reference Standards Catalog Page 2 Catalog Description Current Lot Previous LotValid Use Date CAS NDC Unit Price Special Restriction Container Type 1000441 Abacavir Related Compound B 20 mg 4-25-diamino-6-chloropyrimidin-4-ylaminocyclopent-2-enylmethanol F1L318 F0H283 31-OCT-2013 NA NA 80500 1000452 Abacavir Related.
2
Source:
European Pharmacopoeia Method 222 Degree of Coloration of Liquids European Pharmacopoeia Strasbourg France 1997.
